What is Aldol Condensation?
Aldol condensation occurs in aldehydes having α-hydrogen with a dilute base to give β-hydroxy aldehydes called aldols. This reaction is most commonly known as aldol condensation. If the condensation reaction occurs between two different carbonyl compounds it is called crossed aldol condensation.
Table of Contents
- Aldol Condensation Reaction
- Mechanism of Aldol Condensation
- Crossed Aldol Condensation
- Example of Cross Aldol Condensation
- Types of Condensation
- Frequently Asked Questions – FAQs
Aldol Condensation Reaction
Aldol Condensation can be defined as an organic reaction in which enolate ion reacts with a carbonyl compound to form β-hydroxy ketone or β-hydroxy aldehyde, followed by dehydration to give a conjugated enone. Aldol Condensation plays a vital role in organic synthesis, creating a path to form carbon-carbon bonds.
The general reaction of aldol condensation is

General Aldol Condensation Reaction
One of the common examples for base-catalyzed aldol condensation is stated below in which catalyst generally used is hydroxide ion.
Mechanism of Aldol Condensation
Step-1:
In reverse order, The hydroxide ion deprotonates the aldehyde.
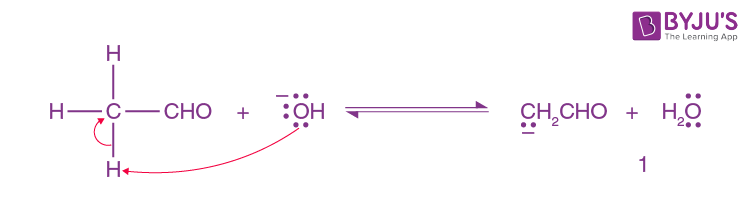 Step-2:
Step-2:
Here Enolate ion 1 adds to the unreacted aldehyde.
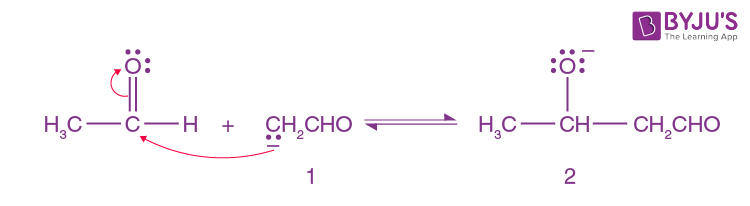 Step-3:
Step-3:
Alkoxide ion 2 is protonated by water.
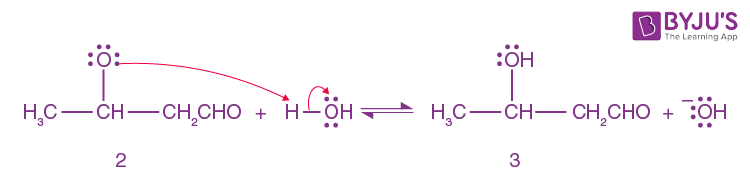 Step-4:
Step-4:
A small amount of aldol is converted into enolate ion (4) by hydroxide ion.
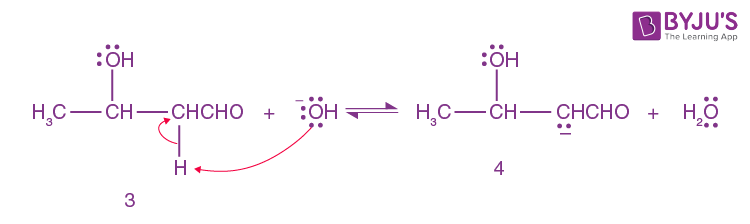 Step-5:
Step-5:
Here Enolate Ion(4) loses a hydroxide ion.
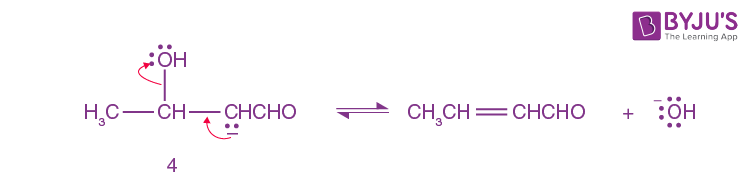
Step 1 to step 3 illustrates the aldol reaction.
Crossed Aldol Condensation
The condensation reaction between two different molecules of an aldehyde or ketone in a protic solvent such as water or alcohol constitutes the crossed aldol reaction. When condensation is between two different carbonyl compounds, it is called crossed aldol condensation. When both aldehydes have alpha hydrogens, both can form carbanions and can also act as carbanion acceptors. Hence a mixture of four products is formed which has little synthetic value.
If one of the aldehydes has no alpha hydrogen then it can act only as a carbanion acceptor. In such a case, only two products are formed. A common substrate for the crossed aldol reaction is an aromatic aldehyde, which has no alpha position. Furthermore, dehydration of the initial condensation product is rapid which leads to the formation of the α, β – unsaturated ketone and prevents the retro-aldol reaction from taking place.
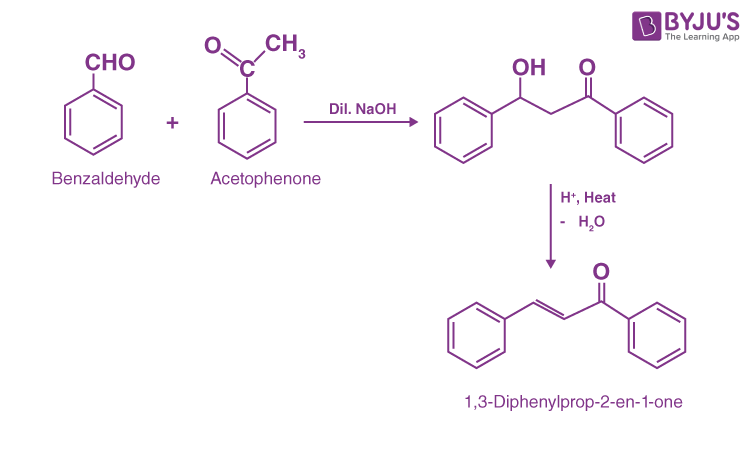
Example of Cross Aldol Condensation:
Reaction between Benzaldehyde and Acetophenone:
The reaction between benzaldehyde and acetophenone undergo cross aldol condensation in presence of dil. NaOH. In this reaction benzaldehyde have no alpha hydrogen but acetophenone have alpha hydrogen so its undergo aldol condensation form β-hydroxy ketone. Furthermore, dehydration leads to the formation of the α, β – unsaturated ketone.
Types of Condensation
It is important to differentiate aldol condensation from various reactions of carbonyl compounds.
- In a case of Perkin reaction, enolate generated by anhydride is aromatic.
- A Claisen condensation contains two ester compounds.
- A Henry reaction contains an aliphatic nitro compound and an aldehyde.
- Dieckmann condensation contains 2 ester groups present in the same molecule, which produces cyclic molecule.
- In Japp–Maitland condensation, water is removed by nucleophilic displacement.
Recommended Video
Aldol Condensation – Aldehydes, Ketones and Carboxylic acids

Frequently Asked Questions – FAQs
What is Aldol condensation?
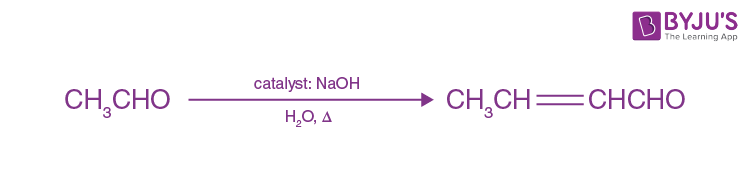
When aldehydes and ketones having at least one α-hydrogen are treated with dilute alkali (which act as a catalyst) they form β-hydroxy aldehydes (aldol) or β-hydroxy ketones (ketol) respectively. This reaction is known as aldol condensation.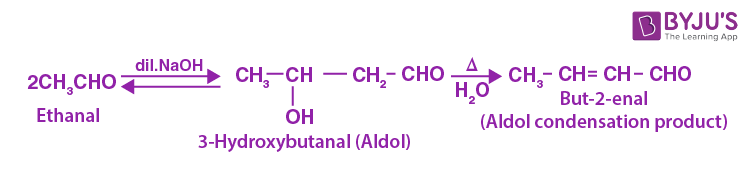
Explain the mechanism of Aldol condensation.
Aldol condensation is an organic reaction in which an enolate ion reacts with carboxyl compound in order to form a β– hydroxy aldehyde or β– hydroxy ketone.
- Hydroxide functions as a base and therefore moves the acidic a-hydrogen producing the reactive enolate ion. This reaction can be seen as an acid-base reaction.
- The aldehyde is attacked at the electrophilic carbonyl carbon by the nucleophilic enolate ion. This attack is a nucleophilic addition reaction and gives alkoxide intermediate.
- The alkoxide deprotonates water molecule, thereby producing hydroxide and the β–hydroxy aldehyde.
Which reference books can be followed to prepare for aldol condensation?
For studying aldol condensation one can follow the NCERT chemistry textbook part-2 for class 12. The chapter named ‘Aldehydes, Ketones and Carboxylic acids’ in this book contains this process.
What is crossed aldol condensation?
Crossed aldol condensation is a variation of aldol condensation in which two dissimilar carbonyl compounds (each containing alpha hydrogens) undergo the condensation reaction together. In such reactions, up to four different products may be formed.
What is the Aldox process?
The Aldox process is an industrial variation of the aldol condensation reaction for the direct conversion of syngas and propene into 2-ethyl hexanol. This product is formed via the hydroformylation of the reactants into butyraldehyde, its subsequent aldol condensation into 2-ethyl hexenal, and the hydrogenation of this intermediate into 2-ethyl hexanol.
For a better understanding of this concept, use the book Organic Chemistry by Morrison & Boyd or reference books such as Organic Chemistry written by Solomons & Fryhle. Solving previous year JEE papers will also be an added advantage on this topic.



Comments